

Reactivity in periodic table series#
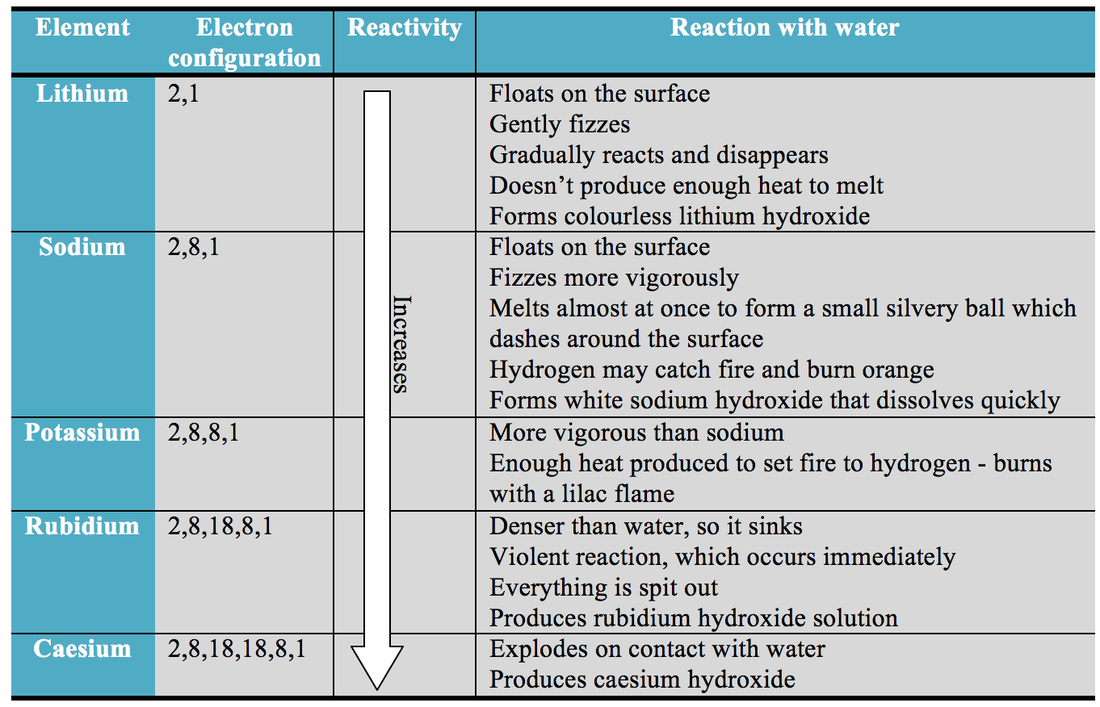
The reactivity series offers a ranking of the metals in order of their reactivity. Caesium, the most reactive metal in the periodic table, reacts extremely violently – hence why it can’t be demonstrated in a classroom! This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water. Lithium fizzes gently, sodium fizzes vigorously, and potassium’s reaction is so energetic it bursts into a lilac flame as it zips across the water’s surface. In this demonstration, small pieces of three different metals from group 1 of the periodic table are dropped into a large bowl of water. Metals have a range of reactivities – to illustrate this, you have to look no further than the classic alkali metals in water demonstration commonly used in chemistry classes. This graphic places a selection of common metals into order of reactivity, as well as showing their reactions with air, water and steam. It’s also a useful tool in predicting the products of simple displacement reactions involving two different metals, as well as providing an insight into why different metals are extracted from their ores in different manners. We hope you find what you are looking for about Reactivity Of Metals In Periodic Table.The metal reactivity series is a commonly taught concept in chemistry, placing the metals, as its name suggests, in order of reactivity from most reactive to least reactive.

As a result, these metals are considered priority metals of public health significance. The actual nature in the adverse reactions of heavy metals depends on their chemical substancespecies and age, and genes. Man exposure to chemical toxins has numerous critical outcomes, which include neurological effects, cardiac ailments, developmental problems, hearing problems, and malignancy. Though chemical toxins in a natural way happen in the environment, anthropogenic routines certainly are a major cause of toxins in the setting. And if you imagined helium was risky, consider this: helium is definitely the minimum reactive of all the respectable fumes. One can use them in arc welding, inflating car tires, and reducing the solubility of pure oxygen for strong-seas divers. Right up until just recently, you might have not thought about the position of noble toxic gases within the Routine Table.
Keep reading to have an clarification of the halogens are, how they're created, and the way they're identified. Halogens are really unusual on the planet and are radioactive. They may be composed of chlorine, bromine and fluorine and iodine, so when on this producing, astatine. These extremely reactive no-metallic components are found in class 17 and make up the 17th line. Halogensġ selection of the regular desk that does not belong from the aluminum loved ones are the halogens. These discoveries ought to stage material professionals towards swap components and advertise much better trying to recycle policies for alloys. The investigation will recognize six critical elements, in which just a few are functionally re-cycled. This investigation may also determine all those materials which are not functionally recycled, and may gather information on end-of-daily life failures, mining companionality, and import addiction. Within the perspective in the recycling of alloys as well as their components, numerous sophisticated metallic alloys include several or more elemental ingredients. Because of their properties, metals are grouped according to their positions in the periodic table. Moreover, these components are given to shed electrons, and will form ions having a beneficial fee or no fee at all. Metals demonstrate numerous attributes, which includes power conductivity, lustre, and malleability. This group involves each precious metals and nonmetals. The primary band of the periodic dinner table makes up the elements with s and p electron layouts. The components of changeover metals are essential with regard to their use within the planet, in addition to their unique components also assist to make clear their recognition amongst scientists. Their least expensive boiling and melting points are linked to no-metals.

The very best melting and boiling things are linked to transition materials. These reactive elements frequently type ores with many other metals and no-alloys. Within the Routine Kitchen table, cross over alloys are normally abundant aspects within the earth's crust. Within the Occasional Desk, alkali metals are called the s-block components. As their reactivity increases, they also become more reactive in water. These are generally present in salts, and have a physique-structured cubic construction. These metals are most reactive when in contact withair and water, or oil. The name alkali metals originates from the Arabic phrase al-qali, meaning ashes.


 0 kommentar(er)
0 kommentar(er)
